Section 4.2 – Chemical Pathology Training Manual University of Cape Town
Section 4.1 – Learning objectives and record of rotations
| Rotation # | Date January 2018 – February 2018 | Initiation |
OBJECTIVES
- Familiarise myself with the laboratory systems, processes and workflow
- Get the know the staff involved and responsible for the various roles
- Understand and practice the functions at the various parts of the lab
- Learn to perform sample reception, triage, handling of STAT samples, urine toppling
- Learn to capture patient data, print labels and label tubes
- Learn to prepare samples as necessary and to load them onto the automated analyser
- Learn to load STAT samples
- Understand the mechanisms and modules on the analyser and what can go wrong
- Read manual SOPs, observe and practise manual assays
- Read electrophoresis SOPs and learn to run and to interpret electrophoresis
- Begin handling clinician queries and learn to troubleshoot pre-analytical queries
- Take on learning tasks proposed by consultants
- Attend academic meetings and seminars and prepare a journal club presentation
- Attend registrar tutorials with consultants
REFLECTIONS
One cannot always get to do everything everywhere. I would like to have spent more time to learn to work the automated chemistry analyser, but this is still the work of the technologists and I have learned a lot from their teachings. It was amazing to learn to know the staff in the lab and build relationships – a good way to find ones feet. I was happy to note that many concepts came naturally to me and I was excited to explore the areas that I didn’t know much about. It was difficult to manage relationships with some of the technologists – but that is understandable as some people in the laboratory have their way of doing things and may be in the way of learning opportinities – especially certain practical tasks. I found my first journal club one of the most daunting experiences ever, however, I enjoyed it.
| Rotation # | Date March – June 2018 | Lab and Biochem Basics |
OBJECTIVES
- Understand the methods used in the laboratory and revise relevant physiology from the 3rd year notes
- Become more familiar with the assays offered at our lab. Learn about the sample requirements for our assays and those that we send away. Become familiar with referral laboratories and where to send specific assays
- Continue learning about the interpretation and verification of routine chemistry results. Find out about extreme values and at what point to investigate further. Troubleshoot suspicious or invalid results
- Interpret protein, lipid and CSF electrophoresis traces and gels and understand when to request further testing. Learn about other causes of monoclonal gammopathy and the limitations of the Friedrichson classification of dyslipidaemias
- Continue to work at the manual bench, becoming very competent at these assays
- Troubleshoot lab issues and lead QC meetings
- Learn about IQC and EQA, how to interpret graphs and reports and how to troubleshoot problems
- Consult with and assist clinicians with diagnoses and testing queries
- Write up and present clinical cases. Read and present research papers at journal club.
- Prepare a talk for the combined metabolic meeting
- Prepare and present undergraduate tutorials
- Continue to attend academic meetings and seminars
WHAT WAS LEARNED
- Guide and teach a new registrar in what has been learned so far
- Understand Lean Management. Observe and quantify the processes in the analytical laboratory at representative times of day and report back to the Lean Management Project Task Team. Be involved in planning of responses to the findings
- IQC reporting was learned and I needed to start leading IQC meetings every fourth week.
REFLECTIONS
Interpretations of electrophores was very interesting in the first few times. I enjoyed following the SOP’s to try to interpret these myself and reading up about the different patterns as well as discovering which patterns are associated with which clinical conditions. A new registrar joined the programme 6 months after I joined, and it was exciting to start teaching a fellow colleague. It was a real difficulty to get accostomed to studying again whilst working full-time. I also enjoyed preparing for undergraduate tutorials – a task in which one likely learns more than the audience you’re giving it to, because it forces you to read up most you can on the topic at hand.
| Rotation # | Date July 2018 – September 2018 | Red Cross Initiation and Exploration |
OBJECTIVES
- Work in the sample reception area: learn how to process samples, create a new entry and refer tests
- Routine analytical lab: become familiar with the processes, systems and workflow in the lab
- Familiarise myself with the routine methods run on the Beckman AU480 analysers
- Participate in daily maintenance procedures on the automated analysers
- To determine whether N-acetylcysteine (NAC) interferes with paracetamol’s measurement, because of a query that my consultant, Prof George van der Watt, received from the toxicology division.
WHAT WAS LEARNED
- Learned to receive samples, data capture, centrifugation, referrals to other labs, handling of special samples, urgent samples and urine samples. Got to know the staff in pre-analytics and developed a good working relationship with them.
- Became familiar with the Beckman AU480 analyser and software. Witnessed and was involved in daily and weekly maintenance. Loaded samples for analysis, made dilutions, performed reruns and loaded STAT samples. Verified results on Trak logged storage details after storing samples.
- Discussed the importance of selecting an analyser that can perform analyses on tiny volumes, as well as methods employed to allow sampling of minimal sample volumes. This being particular to a paediatric laboratory.
- Also observed the use of the osmometer.
- Worked in the IMD lab. Read SOP’s, watched assays being done and learned to perform those assays, including glycosaminoglycan screen and thin layer chromatography (TLC), reducing substances and TLC, sweat testing, free fatty acids, pyruvate, ketones, GALT, citrulline, leucocyte cysteine separation for GC-MS measurement
- I did an experiment to show the interference of NAC on paracetamol measurement
REFLECTIONS
WHAT REMAINS TO BE LEARNED
| Rotation # | Date October 2018– April 2019 | Groote Schuur NHLS Chemistry + Part 1 Exams |
OBJECTIVES
- Gain competency in electrophoresis interpretation.
- Continue learning about our assays and methods, including pros and cons and reasons for the decisions that have been made regarding which analytes to test, and which assays to use.
- Prepare for Part 1 examinations
- Learn to troubleshoot problems picked up in IQC and EQA. Understand EQA programmes and alternatives
- Learn method validation and practice aspects of this in the laboratory
- Learn calculations in laboratory chemistry and practice these (especially important for part 1 examinations and in practice)
WHAT WAS LEARNED
- Gained competency in serum protein electrophoresis interpretation and improved my level of competency in interpretation of urine protein electrophoresis and lipid electrophoresis
- Had the opportunity to observe a CSF electrophoresis for beta-2 transferrin one evening when a colleague was on call, which was a valuable opportunity to become familiar with the instrument and method
- Studied the Westgard rules and started to feel more competent regarding the theory and practice of IQC and EQA and the troubleshooting of violations or poor performance. Felt more involved and interested in meetings due to the improved awareness
- Spent time studying and practicing calculations, which enhanced my understanding and appreciation of some of our methods and results that we release.
- In enjoyed applying maths in the biostatistics, which was applicable to my studies of method validation
- I started engaging more with decisions regarding method selection for the laboratory and diagnostic algorithms e.g. for Cushing’s syndrome
- The focused preparation for the part 1 exams brought a lot of clarity to topics and functions that had previously been neglected in a certain sense, or perhaps not well understood due to lack of insight in the bigger picture, but I still saw room for improvement in this aspect
- Attendance at interdisciplinary meetings and ward rounds, and preparation of seminars and presentations, broadened the holistic view of our role in patient care and the clinical pathway
WHAT REMAINS TO BE LEARNED
• After part 1 exams, I needed to start broadening my knowledge to include non-routine aspects of laboratory practice and areas of special expertise offered by us and other centres
• I needed to deepen my understanding of the routine analytical methods employed by us and others, their benefits and limitations
• I had to start focus more on management topics and issues – which will be covered in a further rotation and a management course attended at Stellebosch University.
| Rotation # | Date May 2019 – June 2019 | Red Cross Continued Rotation |
OBJECTIVES
- Work alongside technologists in the IMD lab
- Read SOP’s for assays run in the manual lab and try to understand the biochemical and analytical principles behind steps in the methods
- Observe assays being performed and assist by performing assays when needed
- Understand GC-MS in more detail and watch and understand maintenance procedures
- Study basic practical lab equipment, principles and techniques
WHAT WAS LEARNED
- Observed metanephrine and organic acid extraction for GC-MS measurement
- Assisted technologists by performing assays and making up buffers and reagents
- Began work on a TSH newborn screening assay for detection of congenital hypothyroidism
- I learned how to do an ELISA, from lots of reading, Youtube videos and articles on the topic, trying to optimize the TSH ELISA which we have ordered from abroad.
- I learned how to use two plate readers and how to program the automated Berthold Tristar2 Multimode Reader LB 942
REFLECTIONS
I enjoyed this rotation as it felt that I’m “making a difference” in a way. I also developed an AutoHotkey script to assist with the transcription of urine and plasma amino acid quantified results from CSV (Excel) to Trakcare as these were still typed in for each patient after the runs – a fairly laborious manual task. I needed to learn various coding features such a looping and parsing files – which I’ve used in further developed scripts and data analyses with quite good success.
WHAT REMAINS TO BE LEARNED
- Better understanding of IMD’s and sufficient knowledge should still be gained to assist witht he interpretation of urine organic acids – especially when the consultant is on leave
- To obtain a broader knowledge, to be able to advise clinicians on the tests which we perform at Red Cross Hospital NHLS
| Rotation # | Date July 2019 – July 2020 | Groote Schuur Hospital NHLS Chemistry Clinical (the COVID stretch) |
OBJECTIVES
- Contact special labs: porphyria, immunology, pharmacology, forensics, toxicology to gain some exposure and knowledge to these areas of laboratory service to which we are not routinely exposed
- Focus on journal articles and reviews to become knowledgeable and up-to-date on the current controversies and conversations about current and new assays or practices
- Attend the Stellenbosch University Laboratory Management course and study laboratory management principles
- Try to complete MMed and get up to date with portfolio work
WHAT WAS LEARNED
- Learning point/area 1
- Learning point/area 2
REFLECTIONS
How did this experience shape you as a pathologist?
What new insights did you have about the job and life of a pathologist?
Did you face any situations relevant to ethics?
What did you learn about yourself as a pathologist?
Etc.
WHAT REMAINS TO BE LEARNED
- Next time you come to this rotation or in general
| Rotation # | 1 August – 17 October 2020 | Red Cross Hospital Chemistry |
OBJECTIVES
- Do Thin Layer Chromatogram
- Osmometer TE calculation and assist with Quality Assurance thereof
- Total Error (allowable) calculations and quality assurance of chromogranin A and Procalcitonin on the Kryptor immunoassay analyser
- Turnaround time calculation (TAT) form a data extraction (“Results Listing”) from Trakcare and assisting with finding a reason for TAT failure on certain samples
- Sweat Test Quality Assurance and EQA programme assessment and advice
WHAT WAS LEARNED
TLC
It was good to do a TLC again. I had done this previously too, but haven’t adequately documented and taken pictures:



The Osmometer problem
The osmometer at Red Cross Hospital lab had broken and needed to be replaced. The part which had broken (a probe) was out of manufacture / not easy to source according the suppliers. It happened just after Groote Schuur Hospital lab had received a new osmometer, hence the older working one from there could luckily be used temporarily. Nonetheless, there haven’t been any TE error calculations done on the new or the old osmometer’s QC readings, which I had done. The technologists suspected that the error on the old osmometer was too high and we needed to decide whether it is worth trying to fix it, or replace it with a new osmometer.
CV (and total error) on PCT and Chromogranin A
The CV of the two analytes above needed to be filled in an reviewed. Data for PCT wasn’t available on the EFLM Biological Variation web site, so a literature search was performed to get an estimate.
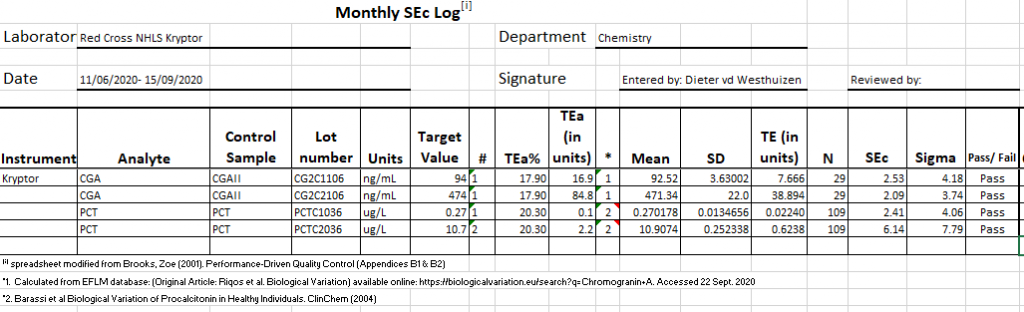

Healthy Individuals. ClinChem (2004).
Turnaround time calculation (TAT)
The two columns in green in the Excel Sheet was created to calculate TAT from a regular results listing extract:
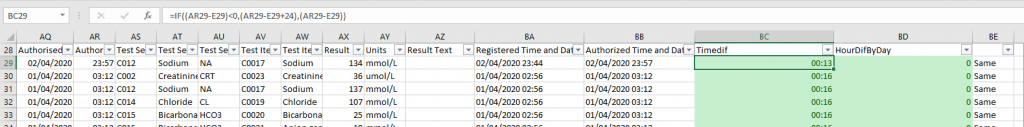
One can play another two or so hours to get the days’ difference in hours and time’s difference in hours into one column, but it’s not particularly easy for me to work with times and dates in Excel.
Formulas used was:
=TEXT(D29,”dd/mm/yyyy “)&TEXT(E29,”hh:mm”)
=TEXT(AQ29,”dd/mm/yyyy “)&TEXT(AR29,”hh:mm”)
=IF((AR29-E29)<0,(AR29-E29+24),(AR29-E29))
=(AQ29-D29)*24
=IF(AQ29=D29,”Same”,”Different”)
I know how to do it in another program, STATA, more precisely, but then you won’t be able to manipulate and count the episodes. To know how to work with times and dates in Excel, a link was found to be very helpful: https://www.ablebits.com/office-addins-blog/2015/06/24/calculate-time-excel/
Also, I know the Technologists at GSH has requested a data extract from Mr. Thomas Papo at CDW when calculating TAT. It works better I think since it’s already nicely formatted and calculated the TAT in a separate column.
Sweat Test Quality Assurance and EQA programme
Since the dilution factor is 1:5, whenever the analyzer gives a result of 1mmol/L more or less than actual, which happens quite often acc. to the bell curve of normal distribution, one’s calculated result differs by 5 mmol/L.
As a result we have now failed our new EQA from Riqas quite far.

To see it in real time on the sheet, change the value in yellow with one unit and observe how the value in blue changes with ~5mmol/L. Our analyzer cannot give a result in decimals.
Ways I could think of to change this issue was:
- use a dilution factor of 1:3 or even smaller or measure the diluted sample few times to get an average.
- use a dilution with an analyte which has no chloride and sodium in it.
In the end it was decided to not do a smaller dilution (as is done by Tygerberg hospital’s Chemical Pathology lab), but rather stick to the method as it is currently. The results are as such that it will not negatively impact patient results. It is not clinically significant and the grey area where sweat testing needs to be repeated according to the guidelines are large enough to not affect the sensitivity due to this resolution issue of 5mmol/L (1mmol/L on the analyser).
We decided to measure the analyte in future neatly on the analyser for EQA purposes and not put it through the same dilution and / or extraction procedure as a normal patient sample would go through.
REFLECTIONS
How did this experience shape you as a pathologist?
What new insights did you have about the job and life of a pathologist?
Troubleshooting is our Job. We need to help with and implement Quality Assurance processes as we go. Quality Assurance is a continuous task with various concepts which needs to be mastered before one gets good at it / understand it.
What did you learn about yourself as a pathologist?
I love statistics. I love maths. I love computer science. I love Chemistry. It inspires me to put the concepts I am learning together to create value and “create and maintain” quality in the lab, if one could say it so proudly.
Other tasks performed:
- Took on interesting cases and researched methods to confirm or support diagnoses, including a toxicology case (GHB poisoning) and metabolic encephalopathy
- Consulted with clinicians asking for relevant clinical information to help make IMD diagnoses and advised about management
- Started to learn to interpret urine organic acid, and plasma and urine amino acid profiles. Interpreted this work when the consultant was on leave – which would be reviewed when he returned.
WHAT REMAINS TO BE LEARNED
- ELISA TSH assay to be perfected / development completed when I graduate
- More about IMD’s in general
- More about GC-MS and the analysis of urinary organic acids – this takes much time and experience to master / learn.
| Rotation # | Date July 2020 – October 2020 | Inherited Metabolic Disease – Molecular Biology Rotation (overlapped slightly with my previous Red Cross Rotation) |
OBJECTIVES
- Revise genetic methods
- Read and understand the genetics lab general SOPs and the TPMT assay SOP
- Watch and learn to perform the TPMT and ENAC assays, paying special attention to precautionary procedures to prevent contamination
- Perform TPMT and ENAC assays independently
WHAT WAS LEARNED
- Read all the relevant SOPs for the molecular lab
- Refreshed the importance of the workflow and avoidance of contamination in the molecular lab
- Gained skills in accurate pipetting and avoidance of contamination whilst working
- Gained knowledge of molecular methods, learned to perform the TPMT and ENaC PCR assays and became familiar with running an agarose gel, as well as using the computer software to document the results, entering them and doing the billing on TrakCare.
- Discussed sequencing and planned to sequence the exons of a gene for a patient I which were discussed from a peripheral private laboratory.
REFLECTIONS
The molecular rotation was likely the most enjoyable of my rotations. It was amazing to learn the basic molecular methods.
I have noticed a shortfall in how planning was done in the IMD laboratory. With regards to outstanding test lists (OTL), I have developed a dashboard where these items can be viewed and planned more efficiently. There are outomated OTL’s generated on TrakCare (07h00 every Friday morning). these are then used to import into the dashboard which organizes the tests outstanding by those which are the longest outstanding. It displays additional info from that on the OTL alone, by using a lookup table to see where the samples are from, as well as calculating by how long the sample has been stored already at our laboratory.
WHAT REMAINS TO BE LEARNED
- I would like to become more proficient in reporting of genetic reports. There are however limited time for us as registrars to thoroughly learn all the post-analytical reporting skills.
- I was also at the molecular rotation during the first wave of COVID, which made personal training challenging.
- Full automation of the IMD OTL dashboard would be great. This is however still an issue with a slow and very limited internet connection which we need to cope with at Groote Schuur Hospital
| Rotation # | Date 19 October 2020 – 31 December 2020 | Management Rotation – Groote Schuur Hospital NHLS |
OBJECTIVES
- Learn about effective leadership skills
- Learn about laboratory organization
- Ethical leadership
- Learn about budgets
- Laboratory Safety
- Preventing and managing conflict
- Use of Quality management tools
- Lean management and quality
- Method validation
- Six Sigma
- Managing a POC service
- Extra-analytical errors
WHAT WAS LEARNED
- Logistic problems which we have in the Western Cape with regards to referral routes, samples being sent from district clinics to sample depots and the organizing of couriers. Although I wasn’t self involved in the process of streamlining the process, I could see the difficulty in organizing this in the NHLS, in an environment where new labs are opened and staff needs to become informed about the changes which is brought about by new labs and new happenings such as COVID.
- EQA, even though being a routine task of us as registrars, was looked at throughout my time in this rotation. We are participating in both Biorad and Riqas’s EQA schemes on some of the assay groups and we have recently moved over to Biorad for our Urinary Chemistry analytes. It is good experience to learn to interpret these charts as they are significantly different and each has its own subtle advantages and disadvantages, although they principally show the same data.
- Human Resource management during the COVID-time was experienced first-hand. We needed to plan actively with the available staff during the pandemic, both to prevent infection of our staff as well as to plan the isolation of infected staff. This involved tasks such as calling infected patients (staff members) and close contacts, keeping schedule of those contacts and being in a position between patients and the laboratory managers, trying to meet both’s needs.
- TAE review: With Dr. Jody Rusch being on leave during some of this time, I was given the task of reviewing our month of December 2020’s TAE sheet. This is a summary of all analytes’ IQC values during the month upon which we comment on the analytes which failed TEA.
REFLECTIONS
How did this experience shape you as a pathologist?
Management skills are not necessarily learnt on paper but by jumping into the deep end. One will not learn management skills effectively by just learning the theory behind it. The management rotation wasn’t necessarily much different than what I feel our task as registrars necessarily are, thus is wasn’t very daunting to me.
What new insights did you have about the job and life of a pathologist?
A very big portion of our job is to assure quality in the laboratory. Managing COVID and it’s related difficulties are not easy and it likely will be here to manage for quite some time.
Did you face any situations relevant to ethics?
None currently
What did you learn about yourself as a pathologist?
I’ve realized once again how important technical skills on a computer is. I’m quite computer literate luckily. It helps with some of the tasks we do in the lab.
WHAT REMAINS TO BE LEARNED
- Preventing and managing conflict
- I would like to implement the Six sigma QC rules for our laboratory on some of the analytes at least
- More of the management of the POC devices we supply to the hospital
| Rotation # | Date 1 Jan – 28 Feb 2021 | Groote Schuur Hospital NHLS clinical rotation and M.Med |
OBJECTIVES
- Report routine serum protein electrophoreses
- Review IQC (Internal quality control) and AON (average of normal) results
- Assist with EQA reports
- Spend time on method development of my M.med (Iohexol clearance by HPLC)
WHAT WAS LEARNED
- Clinical cases were discussed with clinicians in the hospital
- Private pathologists were consulted with some interesting cases
- The failed analytes on our Total allowable error sheet was commented on for December 2020 – a task which I hadn’t done before
- A patient with a significantly elevated lipemic sample was further evaluated with Prof. David Marais
- I learned how to use an Agilent HPLC machine, an Agilent 1260 Infinity, from scratch to finish
- Hands-on training was obtained on working with Agilent’s Masshunter software
- I learned how the data aquisition program works with a diode array detector, the Time of flight (TOF) – Mass Spectrometer (MS) was non-functioning since I started with my project and we had various difficulties and callouts to the supplier.
- I needed to learn some of the maintenance steps of the HPLC, such as column equilibration, reverse flow of columns for cleaning, column storage, basic column care
- I learned how to change a column, changing of tubing, purging, determining the optimal flow rates, chromatographic conditions
- I learned various parts of the sample preparation and optimization thereof to get the highest sensitivity
- I learned how to optimize the chromatographic conditions for the analytes we had used for my method development: iohexol, iomeprol and iothalamate
- I needed to prepare various mobile phases, at various pH values to test each and the effect thereof on my analytes of interest
WHAT REMAINS TO BE LEARNED
Mass spectrometry, further refinement of chromatographic techniques, further understanding of sample preparations / better separation
| Rotation # | Date 1 March – 31 May 2021 | Red Cross Children’s Hospital NHLS Chemistry |
OBJECTIVES
- ELISA TSH assay to be perfected / development completed when I graduate
- More about IMD’s in general
- More about GC-MS and the analysis of urinary organic acids – this takes much time and experience to master / learn.
- Audit of the IMD’s in the past 14 years at RXH
WHAT WAS LEARNED
- Reporting on EQA reports, and doing a Root Cause analysis – commentary was given to assist in future reporting of the EQA reports with possible corrective actions
- Urine organic acid profile reporting was done under guidance of Prof George van der Watt
- Urine amino acid analyses by GC/MS were reported
- Plasma amino acid analyses by GC/MS were reported
REFLECTIONS
I have practiced how to report on EQA reports briefly – exercise makes perfect.
Not everything is always pleasurable – luckily for me, I like statistics and mathematics, so in general I enjoy reporting on statistical calculations and I enjoy to see how different EQA providers function. In this instance, it was noted that we have been provided by EQA material via a middleman who then, likely due to difficult COVID-logistics, struggled to obtain EQA material in time. What appeared to happen was that there was a significantly lower amount of participants in the EQA scheme being supplied by the middleman, hence making thorough statistical analyses less robust.
Less time could be spent on the manual laboratory due to portfolio requirements and studying. The TSH assay which I would like to have spent time on to optimize further, could unfortunately not be optimized thoroughly due to time constraints.
I did start to report more on Urine organic acid profiles and I could perform the reporting of these batched when the consultant, Prof George van der Watt was on leave or busy with other duties, albeit with his input on the reports before being authorized.
Again, I learned that I like to work with statistics. I work efficiently from home and at work. I enjoy working on my personal computer at home because it’s both faster, internet is better and I am more efficient in getting goals done due to less distractions – the downside to this is often that it is frustrating to not have direct access to the laboratory, the samples and the staff – which occasionally is quite a hindrance too.
Possible ethical issues:
Due to COVID and other restrictions, some work needed to be performed from home. The EQA reports were removed from the laboratory and were then sent back to the laboratory via the courier to be filed – likely a possibility for them to become lost in transit – this may be an ethical consideration. Working from home may pose difficult to keep patient’s details truly confidential compared to the work environment.
WHAT REMAINS TO BE LEARNED
- More about IMD’s in general
- More about GC-MS and the analysis of urinary organic acids – this takes much time and experience to master / learn
- Audit of the IMD’s in the past 14 years at RXH (to still be completed)
- We would like to do machine learning to classify these reports from the last 14 years, when this audit is done.