Hyponatremia with a urine sodium measurement
| HOSP # | WARD | Khayelitsha Hospital Emergency Unit | |
| CONSULTANT | Dr. Heleen Vreede | DOB/AGE | 64y female |
Abnormal Result
Serum Sodium of 124 mmol/L
Presenting Complaint
A 64 year old female, presented to the Emergency unit at Khayelitsha Hospital with worsening hyponatremia.
History
The patient had a prior cholecystectomy 6 weeks ago. Histology thereon has shown chronic cholelithiasis but it was complicated with a polyp in the galbladder and adenocarcinoma thereof (completely excised during the cholecystectomy).
Examination
Not available
Laboratory Investigations
| Date | 24/01/2021 | 23/01/2021 | 22/01/2021 | 11/01/2021 | 22/12/2020 | 04/12/2020 | 19/01/2016 |
| Na | δ- 124 L | 129 L | 133 L | 138 | 137 | ||
| Urea | 3,9 | 3,1 | 6,1 | 6,1 | 8,2 H | ||
| Creat | 61 | δ- 66 | 88 | 73 | 86 | 48 L | |
| U Na | • 31 | ||||||
| U K | • 14,9 |
Other Investigations
Liver enzymes were normal at the last measurement (23 January), inflammatory markers normal, COVID-PCR negative on 11/01/2021.
Unfortunately neither the volume status, serum nor urine osmolarity was available on this patient’s history.
Final Diagnosis
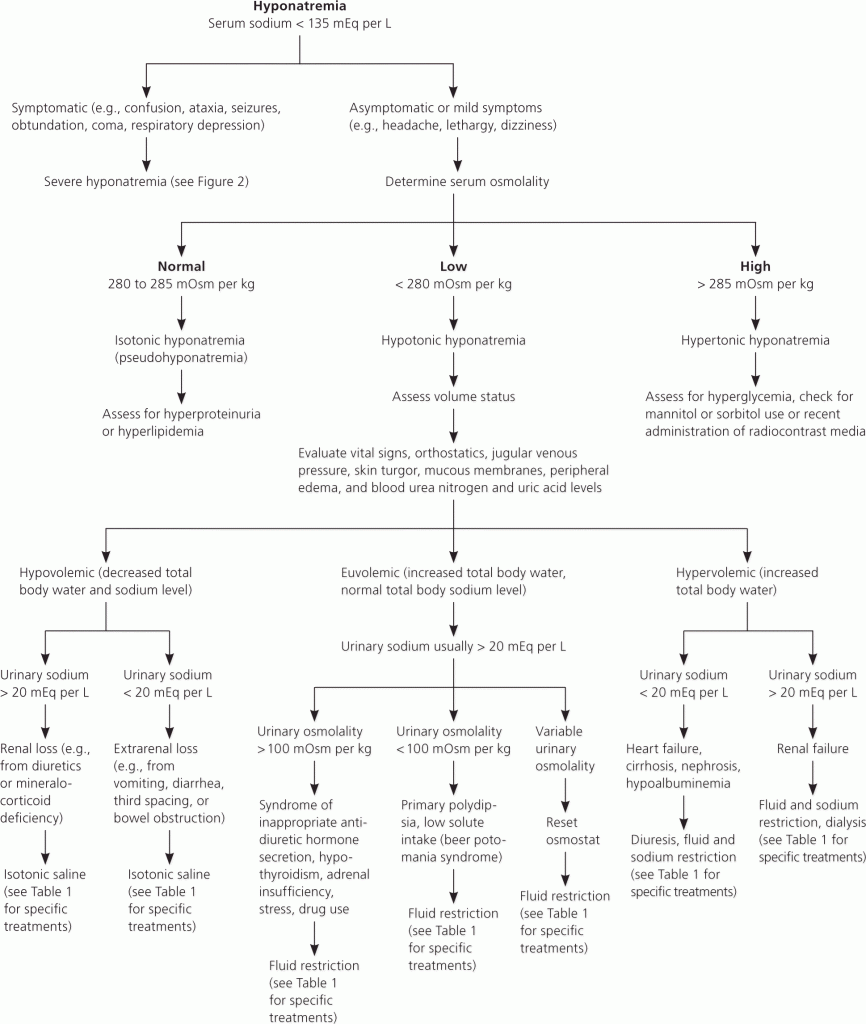
Urinary sodium loss in one form or another. The appropriate response in hyponatremia is to decrease urinary sodium loss. In this patient, the urinary sodium was 31 (should ideally be <20 mmol/L in hyponatremia).
Unfortunately only the urinary sample was sent to our laboratory and it wasn’t possible to assess serum osmolarity.
The volume status was also not available, which is one of the necessities to adequately interpret hyponatremia OR hypernatremia.
Take Home Message
Salt never goes without water – similarly Sodium shouldn’t be interpreted without the volume status of the patient, and the serum (or urine if applicable) osmolarity.
In hyponatremia one expects the kidneys to respond adequately and hold back sodium, hence decreasing urinary sodium (to <20 mmol/L).
Assuming the patient’s osmolarity was low (by estimation formula) the following possibilities ensue:
Patient Hypervolemic: the high urine sodium points toward renal failure.
Patient Hypovolemic: Renal loss (diuretics / mineralocorticoid deficiency)
Patient Euvolemic: Urine osmolarity should be measured:
Urine Osmol >100: SIADH; Hypothyroidism; Hypoadrenalism (although urine Na usually >30); Stress ; Drug use.
Urine Osmol <100: Primary polydipsia / Beer Potomania syndrome
Variable urinary osmolarity: Needs a “reset” of the osmostat by fluid restriction.
Furthermore: Indicators of renal insufficiency in this patient is the increased creatinine above the patient’s baseline of 48 uM. The creatinine has risen to 88 uM on one occasion. Although not above the reference interval for women, this value constitutes a (88-48)/48 = 83% increase in the creatinine and likely will indicate Acute Kidney injury, probably one of the most overlooked causes of morbidity in hospitalised patients in my opinion.