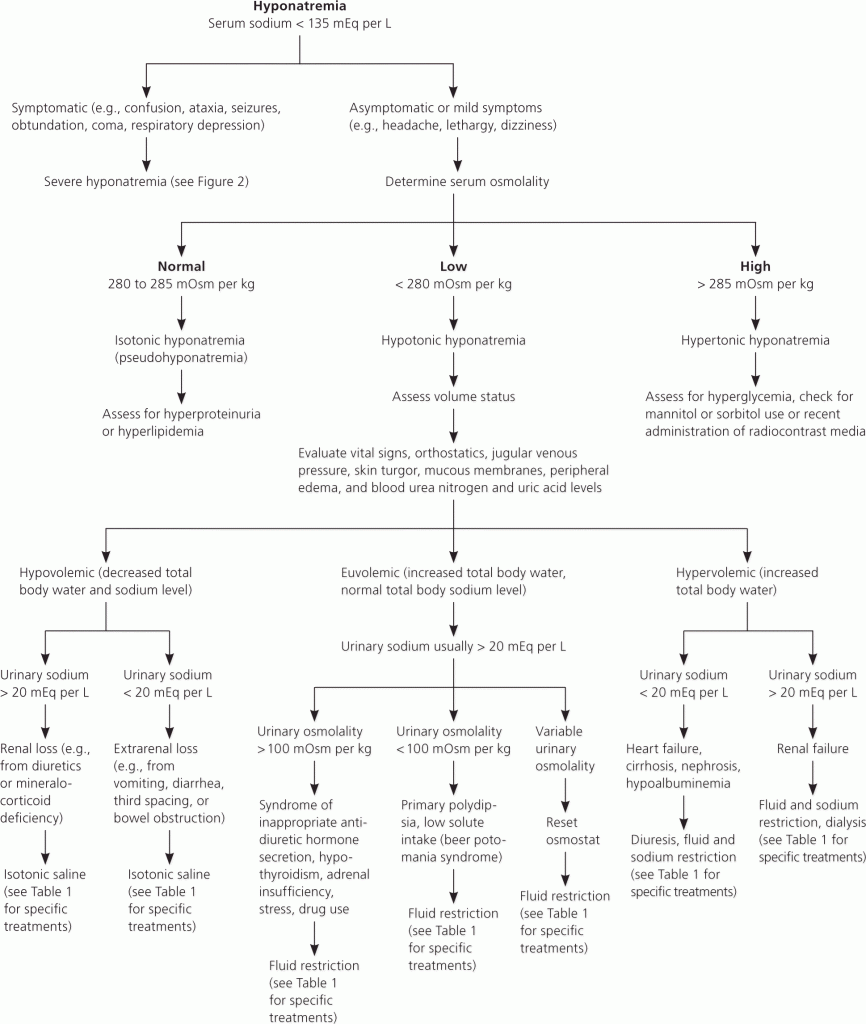
A likely case of thyrotoxic periodic paralysis
| HOSP # | WARD | Internal Medicine | |
| CONSULTANT | Dr. Jody Rusch | DOB/AGE | 21 y male |
Abnormal Result
Potassium of 1.9 mmol/L was found on a blood gas analysis.
Presenting Complaint
Patient presented with a few isolated episodes of muscle weakness. This progressed from 2 weeks before, during the index episode, to become so severe that he couldn’t walk.
History
Patient was given IV potassium + MgSO4 upon which the potassium normalised to 5.5 mmol/L
History of muscle weakness was on and off over the last few months – unable to walk for brief periods of time.
No Family Hx of illnesses / hypokalmeia
No hypertension and no family Hx of hypertension
Patient had sweating more than usual. No other overt Sx of hyperthyroidism.
No medications
The mother had no similar symptoms ever.
The father was unfortunately not involved and not contactable.
Examination
Normal pulses
Small goiter, diffusely enlarger
No cardiovascular system abnormalities
Laboratory Investigations
Potassium upon the current consultation: 4.6 mmol/L
Normal Sodium, Creatinine, calcium, magnesium, phosphate and chloride
Normal pH 7.35
Normal HC03
Suspecting: Hypokalemic periodic paralysis
TSH < 0.01
Free T4: 59 pmol/L
Free T3: 21 pmol/L
TSH-Receptor Antibodies: Increased above the cut-off
Creatine Kinase 749
Other Investigations
The further investigations needed to confirm the diagnosis
Final Diagnosis
Considering the fact that the patient had no renal tubular acidosis, no medication which could cause the low potassium, it was, according to the endocrinologist, likely a diagnosis of Thyrotoxic Periodic Paralysis (TPP).
Patient was placed on Neomercazole and a Beta-adrenergic receptor blocker.
Take Home Message
I wasn’t aware of the condition until this case was brought up to the endocrinology meeting.
Thyrotoxic periodic paralysis is a rare cause of muscle paralysis.
TPP is a disorder most commonly seen in Asian men, is characterized by abrupt onset of hypokalemia and paralysis. The condition primarily affects the lower extremities and is secondary to thyrotoxicosis.
It has been increasingly reported in the USA due to the rise in the immigrant population. Hypokalemia in TPP results from an intracellular shift of potassium induced by the thyroid hormone sensitization of Na+/K+–ATPase rather than depletion of total body potassium. Treatment of TPP includes prevention of this shift of potassium by using nonselective beta-blockade, correcting the underlying hyperthyroid state, and replacing potassium.
It is important for physicians to distinguish TPP from familial hypokalemic periodic paralysis, a more common cause of periodic paralysis in Caucasians. The absence of a family history of paralysis, male sex, presentation in the second to fourth decades of life, and signs of thyrotoxicosis like sinus tachycardia help in the diagnosis of this disorder. Early recognition of TPP is vital to initiating appropriate treatment and to avoiding the risk of rebound hyperkalemia that may occur if high-dose potassium replacement is given.
It is most common in Asian populations – incidence approximately 2% in patients with thyrotoxicosis of any cause.
It has been recognized in Thais, Filipinos, Vietnamese, Koreans, Malaysians, Hispanics, African Americans, and Caucasians. It is characterized by acute onset of severe hypokalemia and profound proximal muscle weakness in patients with thyrotoxicosis.